

The graph of volume vs temperature (in K) is a straight line passing through the origin.įrom the above graph, the volume increases linearly with an increase in the temperature. The graphical representation of Charles' law is demonstrated in the below graphs. Keep reading: Charles' law and absolute zero » Graphical representation According to Gay-Lussac, this could only happen if the gas is an ideal gas. Thus, from the above equation, we arrive at Charles' law, Charles' law and absolute zeroĪs per Charles' law when the absolute temperature approaches zero, the volume of the gas should approach zero. Here, T is the temperature in the kelvin, and T 0 is the temperature in the kelvin equal to 273.15 K. Here, V is the final volume or the volume at T, V 0 is the volume at 0 ☌, t is the temperature expressed in degree Celsius. This statement can mathematically be expressed as: To know more, continue reading on: The equation of Charles' law » Alternative version of Charles' lawĬharles from his experiments concluded that at constant pressure, the volume of a fixed amount of a gas increases or decreases by 1⁄ 273 (now 1⁄ 273.15) times the volume at 0 ☌ for every 1 ☌ rise or fall in temperature. Find the initial volume if the final volume is 2.3 L? Statement: The initial and final temperature of neon is 289 K and 323 K. We can calculate the volume or temperature at any unknown condition if the volume and temperature at a condition is known. Therefore, it obeys the law.įor a given amount ideal gas, V 1, T 1 and V 2, T 2 are the volumes and temperatures of condition 1 and condition 2 at constant pressure. By the same analogy, when the air is cooled, the volume of the air shrinks. Now, the molecules of the air exert more force in the outwards direction on the resting piston, and the air inside the chamber expands. This momentary increases the pressure inside the chamber. the kinetic energy associated with the molecules of the air increases. When the air inside the chamber is heated with a heating source via water, the temperature of the air increases i.e. The figure below consists of a chamber with a piston on it, a water bath to heat the chamber, an electric heating source. Here, k is a constant of proportionality, V is the volume, and T is the absolute temperature.Ĭonsider an enclosed chamber (below figure) with its boundary flexible i.e. The statement can mathematically be expressed as: ExplanationĪs per the law, the volume of a gas is directly proportional to its temperature for a fixed amount of the gas at constant pressure. Gay-Lussac confirmed and extended the results of Charles and credited the discovery to him.
But his discovery remained unknown to the world until Joseph Louis Gay-Lussac in 1802 published the discovery. When the temperature of the balloons was raised, the volume of each increased by an equal amount. He conducted his experiment on five balloons. In his experiments, he observed that the volume of different gases would increase by the equal volume when subjected to a rise in temperature. It was around 1787 when Charles studied the behaviour of gases with temperatures. Jacques Alexandre César Charles was a scientist, inventor, and balloonist. This law is named after French scientist Jacques Charles. It is a very important law studied in chemistry and physics along with Boyle's law, Gay-Lussac's law, and Avogadro's law.Ĭharles' law states for a fixed amount of an ideal gas its volume is directly proportional to its temperature at constant pressure. The law describes the relationship between the volume and the temperature of a gas. We measured the volume over a 40 ☌ range, and we are extrapolating backwards by seven times that range.Charles' law is also known as the law of volumes. It is no surprise that the values differ so much. Thus, the experimental value of absolute zero is -280.6 ☌ We want to find the value of T that gives V = 0. My calculator tells me that the equation is If you use a computer or a calculator, you can tell it to calculate the equation for the line that best fits all the points (the regression line).

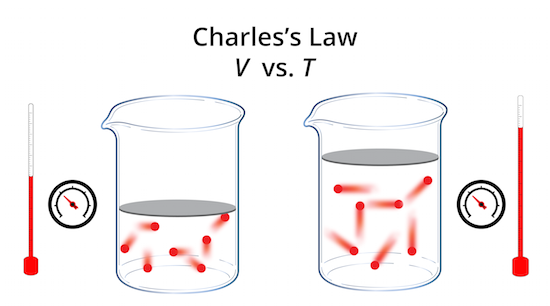
This gives you the temperature at which the gas theoretically has no volume (i.e, absolute zero). If you plot the data manually, you then extend the line backwards until it crosses the horizontal axis at about -270 ☌. You should get a graph that looks something like the one below. You could do this by hand, but it is more convenient to use a computer or calculator to do the job for you. You would then plot a graph with temperature as the independent variable (along the horizontal or x axis) and volume as the dependent variable (along the vertical or y axis). So you would do an experiment in which you measure the volume of a gas at various temperatures. Charles' Law examines the relationship between the volume of a gas and its temperature.


 0 kommentar(er)
0 kommentar(er)
